Step 4: Identify the compensatory process (if one is present)
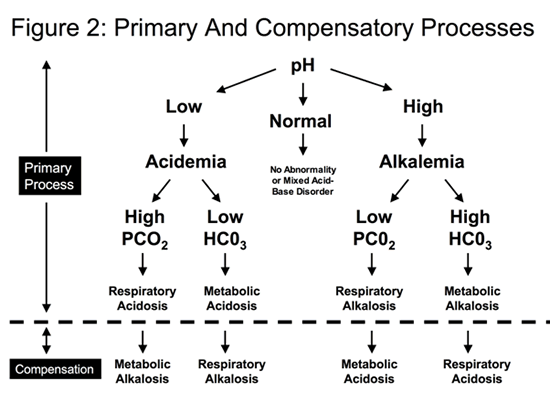
In general, the primary process is followed by a compensatory process, as the body attempts to bring the pH back towards the normal range.
- If the patient has a primary respiratory acidosis (high PCO2 ) leading to acidemia: the compensatory process is a metabolic alkalosis (rise in the serum bicarbonate).
- If the patient has a primary respiratory alkalosis (low PCO2 ) leading to alkalemia: the compensatory process is a metabolic acidosis (decrease in the serum bicarbonate)
- If the patient has a primary metabolic acidosis (low bicarbonate) leading acidemia, the compensatory process is a respiratory alkalosis (low PCO2 ).
- If the patient has a primary metabolic alkalosis (high bicarbonate) leading to alkalemia, the compensatory process is a respiratory acidosis (high PCO2 )
The compensatory processes are summarized in Figure 2.
Some examples of working through this step of the process›
Important Points Regarding Compensatory Processes
There are several important points to be aware of regarding these compensatory processes:
- The body never overcompensates for the primary process. For example, if the patient develops acidemia due to a respiratory acidosis and then subsequently develops a compensatory metabolic alkalosis (a good example of this is the COPD patient with chronic carbon dioxide retention), the pH will move back towards the normal value of 7.4 but will not go to the alkalemic side of normal This might result in a pH of 7.36, for example but should not result in a pH such as 7.44 or another value on the alkalemic side of normal. If the pH appears to "over-compensate" then an additional process is at work and you will have to try and identify it. This can happen with mixed acid-base disorders, which are described further below.
- The pace of compensation varies depending on whether it is respiratory or metabolic compensation. Respiratory compensation for primary metabolic disturbance is almost immediate. For example, if someone infused hydrochloric acid through an IV and gave the patient a metabolic acidosis, the patient would rapidly begin hyperventilating and generate a respiratory alkalosis that would move the pH back towards normal. Metabolic compensation for primary respiratory abnormalities, however, is slow and may take several days. For example, if someone travels to high altitude and begins to hyperventilate due to the low oxygen levels in the atmosphere, initially there will be no metabolic compensation and they will have a high pH. Over several days, however, metabolic compensation will occur and the pH will return back towards normal.
- Despite the compensatory mechanisms, the pH may not return all the way to normal. For example, consider the following arterial blood gas in a patient with acute toluene toxicity: pH 6.95, PCO2 9, HCO3- 2. This is a primary metabolic acidosis but despite a huge degree of hyperventilation and a marked compensatory respiratory alkalosis, the pH still remains very low. In other situations (e.g., a compensated respiratory acidosis in a patient with obesity hypoventilation, or the compensated respiratory alkalosis in a pregnant woman), the pH will return closer to the normal range.
- What may appear to be a compensatory process may not actually represent true compensation. For example, consider a patient who develops an acute respiratory acidosis (large rise in the PCO2). Even though this is an acute process and renal compensation has not occured, the bicarbonate value may read 27 on the ABG. This appears elevated and would suggest metabolic compensation is starting to occur but may, in fact, not represent true compensation. How does this occur? Remember that a key relationship that governs acid-base physiology is the following:
H2O + CO2 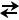 HHCO3- HHCO3- 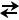 H+ + HCO3- H+ + HCO3-
If there is a large rise in the PCO2 , this equation will shift towards the right and the levels of bicarbonate will transiently increase. Similarly, a large fall in the PCO2 would shift the equation to the left and the bicarbonate would transiently decrease. How can you figure out whether the observed changes in bicarbonate represent true compensation or changes due to this relationship? The answer lies in another value you will see reported with the arterial blood gas—the base excess. In general, as you may recall from respiratory physiology, the base excess is defined as the difference between the patient’s HCO3-
after correction to a pH of 7.4 by a change in the PCO2 and the normal HCO3-
at pH 7.4. It can be used in the following manner to interpret changes in the HCO3-
levels.
- If the base excess is between –2 and +2 then the observed changes in bicarbonate are due to movement based on the equation above and there is no metabolic acidosis or alkalosis.
- If the base excess is less than –2, then there is a metabolic acidosis, which may be the compensatory process. Another term for this is a base deficit.
- If the base excess is greater than +2, then there is a metabolic alkalosis, which may be the compensatory process.
Be aware that if the base excess is less than -2 or greater than +2, and therefore, a metabolic process is present, the base excess value itself does not tell you whether this is a primary process or a compensatory process. It only tells you a metabolic process is present. You still need to work through the steps described here to tell whether it is the primary process or the compensatory process
- What appears as a lack of compensation may actually represent an acute process on top of a chronic process. In some cases, patients may live in a chronically compensated state of acid-base disturbance and then deviate from that state due to an acute problem. The best example is the patient with very severe COPD who might have chronic respiratory acidosis with metabolic compensation at baseline. The arterial blood gas for this patient might look like the following:
- pH 7.35
- PCO2 55
- PO2 70 HCO3- 32
Now, suppose the patient develops a severe exacerbation and comes into the ER.
At that point in time, an arterial blood gas may show
- pH 7.25
- PCO2 65
- PO2 62
- HCO3- 33
The PCO2 is now 10 mm Hg higher than before and the pH is now much further away from 7.40. If you didn’t have the initial blood gas for comparison, you would say this patient has a respiratory acidosis with only partial compensation. However, knowing the prior acid-base status, it would be more correct to say this patient has an acute-on-chronic respiratory acidosis. The acute worsening of his COPD led to a rise in the PCO2 but the bicarbonate has not risen further from baseline and the pH has not moved back towards normal because the kidneys have not had time to compensate for the acute change in the patient's condition.
 Top of Page Top of Page
Step 5: Determine if a Mixed Acid-Base Disorder is Present.
The 4 steps outlined above allow you to fully classify the patient’s acid-base status in the majority of cases. In such cases there is a primary abnormality and, possibly, a compensatory process. There are occasional situations, however, where patients may have more than one disorder going on at the same time. For example, it is possible for patients to have a concurrent metabolic acidosis and metabolic alkalosis or a concurrent elevated anion-gap acidosis and non-anion gap acidosis (The only combination you cannot have is a combined respiratory alkalosis and acidosis as it is impossible for the patient to hyper- and hypoventilate at the same time). These situations are referred to as mixed acid-base disorders.
One way to determine if a mixed acid-base disorder is present is to calculate the anticipated response to the primary abnormality. If the actual response deviates from this value then you know an additional process may be at work. There are equations for example, that stipulate that the bicarbonate should change by a certain amount in response to a chronic respiratory acidosis. The problem is that these equations are difficult to remember and, as a result, are tedious to apply in the acute setting. The nomograms that are often presented in respiratory physiology course are also difficult to use, as well as locate, when you need them.
An alternative approach is to calculate what is referred to as the Delta-Delta. This approach, which gives you a sense of whether the body is holding onto or losing more bicarbonate than you would expect simply based on looking at the pH, serum anion gap (SAG) and the bicarbonate values, can be done as follows:
- Calculate the Delta Gap: Measured SAG – Normal SAG (12)
- Calculate the Delta Delta: Add the Delta Gap to the measured bicarbonate (from the chemistry panel)
- Compare the Delta Delta to a normal bicarbonate (22-26):
- If the Delta Delta <22, the patient is losing bicarbonate somewhere and there is a non-gap acidosis. If you found a non-gap acidosis (either the compensatory or the primary process) in the initial steps above, then the acidosis you identify here represents the same process. If however, you did not identify an acidosis in the earlier steps or you identified a gap acidosis in the initial steps, then this last step reveals the presence of an additional non-gap acidosis.
- If the Delta Delta >26, the patient is holding onto bicarbonate and there is an additional metabolic alkalosis. If you found an alkalosis (either the compensatory or the primary process) in the initial step above, then the alkalosis you identify here represents the same process. If however, you identify an acidosis in the initial steps and then this last step reveals the presence of alkalosis, you have found an additional metabolic process.
Be aware that for these calculations to work, the chemistry panel and blood gas must have been drawn at roughly the same time. If, for example, you obtain a blood gas on a patient during a code on a patient at 6PM, you cannot use the morning chemistry panel from 6AM to do these calculations.
Examples of how to work through this last step of the process›
 Top of Page Top of Page
< Previous: A
Step-wise Approach to Interpretation | Next:
Generating Differential Diagnoses
|