A Step-Wise Approach to Acid-Base Status Interpretation
As with reading an electrocardiogram or a chest x-ray, it is important
to use a system when reading arterial blood gases. Adhering to a system
will allow you to identify the primary and compensatory process and any
additional disorders that may be present.
A suggested step-wise approach for reading an arterial blood gas is as
follows:
- Examine the pH and comparing it to the normal range
- Identify the primary process that led to the change in pH
- Calculate the serum anion gap
- Identify the compensatory process (if one is present)
- Identify if any other disorders are present or there is a mixed
acid-base process
Each of these steps is described below in greater detail. After working
through these steps, you should be able to give a one- or two-sentence
synopsis of the patient’s acid-base status such as "This patient has a
primary respiratory acidosis with a compensatory metabolic alkalosis."
As you go through this process, try not to lose track of the clinical
scenario that led to the blood gas being drawn in the first place. You
will use the results and their interpretation to help you figure out what
is going on with the patient. In addition, you should always ask if the
results make sense in light of what you know about the patient’s case. If
the results do not make sense, either your interpretation was wrong or
there may be some additional processes at work that were not recognized on
the initial analysis.
With that in mind, the main steps in interpreting an arterial blood gas
in greater detail are presented below.
Step 1: Examine the pH and compare it to the normal range.
As noted above, if the pH is low, the patient has an acidemia. If the
pH is above this range, the patient has an alkalemia. Be aware that
patients can have mixed metabolic disorders (e.g., concurrent metabolic
acidosis and alkalosis) that can give them a pH in the normal range. This
will be discussed further below.
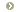 Top
of Page
Top
of Page
Step 2: Determine the primary process that led to the change in the
pH:
For a patient with a low pH (acidemia)
- If the PCO2 is elevated (> 44), the primary process is a
respiratory acidosis
- If the HCO3- is low (< 22), the primary process is a
metabolic acidosis
For a patient with a high pH (alkalemia)
- If the PCO2 is low (< 36), the primary process is a
respiratory alkalosis
- If the HCO3- is high (> 26), the primary process is a
metabolic alkalosis.
This framework is depicted in Figure 1.
Some examples
of how to work through this first step in the process›
 Top
of Page
Top
of Page
Step 3: Calculate the serum anion gap (SAG)
Serum Anion Gap (SAG) = Na+ - (Cl- + HCO3- )
You should use the bicarbonate from the chemistry panel for this
calculation. If this value is elevated (> 12), the person is deemed to
have an "elevated anion gap." This implies that the patient has a primary
elevated serum anion gap metabolic acidosis regardless of what other
abnormalities you identify or what else is happening with the pH and
bicarbonate. There must be an additional disorder because the body
does not generate an anion gap in order to compensate for a primary
respiratory disorder.
Be aware, however, that an elevated anion gap acidosis may not be the
only primary process. For example, patients with salicylate intoxication
may have a primary respiratory alkalosis and a concurrent primary elevated
anion gap acidosis at the same time.
An example of
how to work through this situation›
You should also note that the normal anion gap is affected by the
patient's serum albumin level. As a general rule of thumb, the normal
anion gap is roughly three times the albumin value. By way of example, for
a patient with an albumin of 4.0, the normal anion gap would be 12. For a
patient with chronic liver disease and an albumin of 2.0, the upper limit
of normal for the anion gap would be 6. Other people propose that the
ceiling value for a normal anion gap is reduced by 2.5 for every 1g/dL
reduction in the plasma albumin concentration.
 Top
of Page
Top
of Page
< Previous: How the
Data Are Presented | Next: Step 4:
Identify the compensatory process (if one is present)