How the Data Are Presented
While the laboratory will always label each value in the arterial blood
gas results, it is not uncommon for residents, fellows and attending
physicians to either write or state the results without labeling each
value. For example, rather than stating: "the pH is 7.40, the PCO2 is 40,
the PO2 is 85 and the HCO3- is 24" they may simply state or write:
"7.4/40/85/24."
If ABG results are presented in this manner, by convention, they will
be written or spoken in the following order:
pH 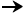 PCO2
PCO2 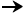 PO2
PO2 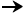 HCO3-
HCO3-
Before you get started…. Make Sure the Numbers Are Consistent
Before you do you acid-base interpretation, it is important to do a
little troubleshooting and make sure there are no measurement errors with
your blood gas results.
There are two things you should do.
First, make sure it is an arterial sample and not a venous sample. The
best way to do this is to observe how the blood comes back into the blood
gas syringe as the sample is drawn. Pulsatile flow is seen with an
arterial sample but would be lacking with a venous sample. Similarly,
arterial samples usually fill the syringe quickly, while venous samples
move much more slowly into the syringe. You cannot always rely on the
color of the blood to tell you it is arterial because a very hypoxemic
patient will have dark, "venous-appearing" blood. If you did not see the
sample as it was drawn into the syringe, you can use the PO2 as
a guide. If the patient was not very hypoxemic when the blood gas was
drawn but you get a very low PO2 with the results (30s-40s), it
is likely that you have a venous sample. This tactic is a bit harder to
use when the patient is very hypoxemic when the sample is drawn.
Second, you should make sure there are no measurement errors. A simple
way to do this is to compare the bicarbonate value from the blood gas (a
calculated value) with the bicarbonate from the chemistry panel (a
measured value). They are not always exactly the same but they should be
close to each other. This only works, however, if your chemistry panel and
blood gas were measured at roughly the same time. You cannot do this if
the samples were drawn many hours apart.
A more thorough approach is to see if there is consistency between the
blood gas and the chemistry panel using the Henderson-Hasselbach equation.
The equation is used to calculate the pH you would expect based on the
measured PCO2 and HCO3-. This pH is then compared to
the measured pH. If the values are similar, your sample is valid. If the
values are far apart, there may be a measurement error.
Because no one can easily remember the full Henderson-Hasselbach
equation, there is a modified process that can be used instead. This
modified process is as follows:
- Calculate the hydrogen ion concentration using a modified
Henderson-Hasselbach equation: [H+] = 24 x PCO2 / HCO3-
- Use the calculated [H+] to determine what the pH should be. pH is a
function of the hydrogen ion concentration (pH = -log [H+]) but rather
than doing the calculation you can refer to the following table: